
CAS number: 74578-69-1
Molecular formula: C18H16N8Na2O7S3
molecular weight: 598.5436
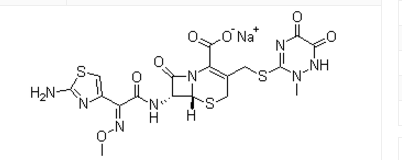
Chemical structure:
| Product Name | PHARMACEUTICAL ALLOPATHIC RAW MATERIAL FOR INDUSTRY Ceftriaxone Sodium Sterile USP | |
|
Shelf life |
3 years | |
| TEST ITEMS | SPECIFICATION | RESULT |
| Description | white to yellowish-organge crystlline powder. | Almost white crystalline powder |
| Identification |
|
|
| By IR | Meets the requirements. | Complies |
| By HPLC | Meets the requirements. | Complies |
| Tests for sodium | It gives reaction of sodium | Positive |
| Test |
|
|
| Crystallinity | Meets the requirements. | Complies |
| pH | 6.0~8.0 | 6.5 |
| Impurity A | Deacetylcetotaxime lactone≤0.5% | Not Detected |
| Impurity B,C | 7-Aminocephalosporanic acid≤0.5% | 0.01% |
| Impurity D | Ceftriaxone triazine analog≤1.0% | 0.09% |
| Impurity E | Ceftriaxone benzathiazoly oxime≤0.2% | Not Detected |
| Impurity F | Deacyl ceftriaxone≤0.5% | 0.07% |
| Impurity G | Ceftriaxone 3-ene isomer≤0.3% | Not Detected |
| Impurity H | Ceftriaxone E-isomer≤0.5% | 0.02% |
| Any individual | ≤0.2% | 0.04% |
| Unknown impurity |
|
|
| Total impurities | ≤2.5% | 0.3% |
| Water(By KF) | 8.0%~11.0% | 9.3% |
| Methanol | ≤0.3% | Not Detected |
| ≤0.5% | Not Detected | |
| ≤0.041% | Not Detected | |
| ≤0.5% | Not Detected | |
| ≤0.06% | Not Detected | |
| ≤0.5% | Not Detected | |
|
Partieless≥10μg/mg:NMT 6000 part/g |
660 | |
| Partieless≥25μg/mg:NMT 600 part/g |
|
|
| <0.20EU/mg | Complies | |
| It complies with the test for sterlity | Complies | |
| Informative | 0.54g/ml | |
| Informative | 0.72g/ml | |
| ASSAY |
|
|
| Ceftriaxone on anhydrous basis | ≥795μg/mg | 932μg/mg |
| Storage Condition Preserve in airtight,tamper-proof container;in a dry place at a temperature not exceeding 20℃. | ||
|
RESULT: THE RESULT CONFORMS TO USP38、QS-CO11014(USP)-01. |
||